Newsbreak: Genetic Editing moves the World towards New Cure for Sickle Cell Disease
Abraham Ariyo, M.D.
November 10, 2023.
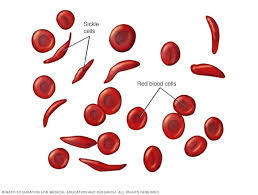
Sickle Cell Disease is an inherited blood disorder. First described in 1910, it afflicts millions, causing pain, suffering, and premature deaths among afflicted individuals. It is prevalent among Africans and African Americans, in whom the burden weighs heavily.
A US-based biotechnology company recently unveiled a novel technique that promises to cure the disease completely. This new approach aims to eradicate the anomaly in the person with the disease.
On October 31, 2023, in Dallas, Texas, National Public Radio (NPR) featured Victoria Gray, a 38-year-old woman with the disease from Mississippi, who recently underwent the procedure. She has had Sickle Cell all her life with devastating and crippling adverse effects from it. She was always sick with pains and aches in her joints and over her body: always in and out of the hospital, could not take care of herself or her four children, and could not hold a job. This description was typical of most patients with this disease forty years ago when we were Medical Students.
In 2023, things are changing. First, after consenting to this experimental therapy, cells (DNAs) were extracted from her bone marrow. They then took these DNA samples to the laboratory and performed genetic editing. The process involved identifying and removing the gene coding for Sickle Cell from her DNA sequence.
The newly edited DNA sequence cells, called supercells, now lack the gene that codes for sickle cell formation. Specifically, the translation of her new gene sequence coding for red blood cell (RBC) protein will form new cells that will not sickle under any condition, a previous hallmark of sickle cell disease.
After the gene editing completion, billions of these supercells got infused back into her bone marrow. The risk of rejection was null because they were her cells, although they had changed. She tolerated the procedure well. Subsequently, her body recognized the new DNA sequence and translated it into producing new normal red blood cells. Her new RBCs did not sickle and carried oxygen at optimal (maximum) capacity.
Mrs. Gray stated that she feels cured. She feels normal: able to take care of herself and her children, not sick anymore, not needing to visit the hospital, and able to work and hold a job like regular people. She is grateful for this opportunity to have her disease corrected and now lives an everyday life. The Food and Drug Administration has yet to approve this treatment for everyday use because they do not know the long-term consequences of this gene editing. However, knowing there is a potential for a cure for this debilitating disease and that a cure may be around the corner for 20 million people afflicted by this ailment worldwide symbolizes a significant milestone and a source of hope for humanity.
Abraham Ariyo, M.D.
HeartMasters, Dallas, Texas.
